
News Archive
You will find the latest information about our company and industries here. Please feel free to contact us with any questions and suggestions relating to our topics or content!

Here we go! Much more space for DR. BRILL INSTITUTES in Hamburg
The first movers started work today at our main site in the north of Hamburg. We will of course remain at our location in the beautiful Hummelsbüttel district, but will be moving into great new premises in the immediate vicinity. We are really looking forward to it! And of course we will keep you up to date with any further news about the move.

Co-lecture by Bernd Daehne at the Sustainable Shipping Conference 2024
In April 2024, our Institute for Antifouling and Microbiology will be represented at the Sustainable Shipping Conference 2024, the "6. Bremer Kongress für Nachhaltigkeit der Schifffahrt", with a co-lecture entitled "Antifouling – Notwendigkeit und Perspektiven" (Antifouling - Necessity and Perspectives). Speakers: Dr. Burkard Watermann, LimnoMar, and Bernd Daehne, Dr. Brill + Partner GmbH Institute for Antifouling and Microbiology - as part of Workshop 2: Biofouling. The congress will take place in German on 22-23 April 2024, in the city of Bremen. We would be very pleased about your participation! All information on the content and for your free registration can be found under the following link:
Sustainable Shipping Conference 2024
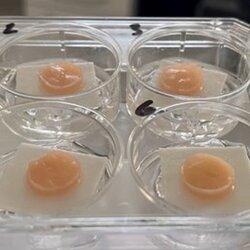
New wound infection model based on artificial 3D skin
In the brandnew paper Wiegand et al. 2024 (International Wound Journal, in press) we are presenting parts of our data from our InVitroWound project which was granted by BMBF call "Alternatives to animal testing". In cooperation with PD Dr. Cornelia Wiegand from the University Medical Center Jena we developed an infection wound model based on artificial 3D skin. In this assay we can test antimicrobial wound are products without animals on their antimicrobial, biocompatibility as well as healing performance. This is a milestone in practice-like in-vitro testing with new endpoints.
If you are interested in the data or performing such studies please feel free contacting us.
UPDATE: On 13 March 2024 the paper "A standardized wound infection model for antimicrobial testing of wound dressings in vitro" hat been published. Link: here

News from the expert groups
DR. BRILL INSTITUTES is engaged in several expert groups to contribute continuously to the scientific know-how for patient and environmental safety.
In February Dr. Florian H. H. Brill participated as Convenor of the Working Group 5 in the high-level CEN and CENELEC TC 216 plenary meeting with the who is who of efficacy testing of disinfectants and antiseptics in Lille hosted by Laboratoires Anios with Sophie Loeffert-Frémiot and led by the chairwoman Christine Roques. A number of important strategic decisions were made.
In March Dr. Florian H. H. Brill participated in the Working Group 3 meeting of CEN TC 216 in Milano. He reported from his strategic working group 5 as well as the on-going projects for developing a phase 2 step 2 test for surface disinfection with mechanical action for WG 3 applications e. g. in food and feed area with an innovative automated application procedure.
Also, in March our surface disinfection expert Anna Ulatowski will report in the German mirror committee for WG 2 and 3 on the projects of PAS 2424 into an EN method and her development of a phase 2 step 2 surface disinfection test with mechanical action using an automated application procedure. Anna's Method for better standardization of drying microorganisms was published as part of the new EN 13697 in January.

New method to test domestic laundry detergents against viruses
With the paper Konkol et al. we aimed to develop a method to assess the virucidal performance of domestic laundry in a lab-scale washing machine (Rotawash) based on EN 17658 in Cooperation with Henkel. The method developed was led by our joint PhD student Justyna Konkol in cooperation with the Henkel team led by Dr. Mirco Weide in Düsseldorf, our Dr. Brill Virus experts in Bremen Dr. Britta Becker and Dr. Dajana Paulmann as well as from our cooperation partner Professor Dr. Eike Steinmann from Ruhr-University Bochum, Professor Dr. Andreas Dotzauer from University Bremen as well as our Dr. Brill + Prof. Bockmühl lab director Dr. Britta Brands from Kleve.
With this great team approach we are now prepared to test domestic laundry detergents against e. g. Vaccinia and Norovirus. If you are interested or have questions please contact us. Link to publication: Here

Seminar MDR MEETS HYGIENE - Cooperation between NSF Prosystem and DR. BRILL INSTITUTES
From 18 - 19 April 2024 you can find out from experts which aspects of hygiene need to be taken into account in the context of the Medical Device Regulation MDR. Together with NSF Prosystem, a competent partner in medical technology, DR. BRILL INSTITUTES is organizing a seminar on this topic. We look at various factors that play a role in placing reprocessed medical devices on the market so that you have an improved understanding of the reprocessing validation processes. So, if you produce reprocessable medical devices, are a supplier to manufacturers of medical devices or generally deal with the topic in the context of quality management or regulatory affairs, we look forward to welcoming you to Hamburg after registering using the following link: here (The seminar will be held in German.)

Presentation by Bernd Daehne at the Blauer Engel webinar
Our Institute for Antifouling and Microbiology will be represented by Bernd Daehne as a speaker at the webinar in German language "Blauer Engel für Unterwasserbeschichtungen und andere Bewuchsschutzsysteme (DE-UZ 221)" (Blauer Engel for underwater coatings and other anti-fouling systems (DE-UZ 221)". His topic: Presentation of the award criteria "Effectiveness". The webinar will take place on 21 March 2024 and we look forward to your participation! All information on the content and your free registration can be found under the following link: Blauer Engel webinar

Dream team brings regulatory expertise in-house
With a real "dream team", Dr. Brill Institutes will be able to offer customers from industry its own Regulatory Service in the future. Heike Schimmelpfennig and Dr. Gunnar Kleist, each with two decades of regulatory experience in different areas of industry and public authorities, offer a 360-degree view of chemical testing and approval of biocides (active substances and products).
"With our wealth of experience from different perspectives, we are unique on the market," explains Dr. Gunnar Kleist confidently. "We offer our customers holistic project management, which can take five years from the first kick-off meeting to approval, depending on the process." The two are more than trustworthy sparring partners in a process that demands complete transparency and honesty from the customer.
Thanks to its many years of experience, the Regulatory Services team is also able to master critical situations at short notice when companies get lost in the complex European chemicals legislation or miss important deadlines, which can sometimes threaten their existence. However, both prefer to work with the customer from the start of the process in order to avoid precisely such crisis situations.
"In our role, we also see ourselves as a mediator between the needs of industry and the expectations of the authorities," says Heike Schimmelpfennig. "We know what the authorities want, but we also know the needs of industrial customers."
Heike Schimmelpfennig has ten years of experience in the environmental risk assessment of chemicals in both a consultancy firm and an EU regulatory authority. Dr. Gunnar Kleist contributes his more than 20 years of corporate experience in industrial research, test method development and Regulatory Affairs. Their joint core competence is to incorporate all relevant information from their profound knowledge of internal company processes into an approval application in such a way that a smooth and successful completion of the entire procedure is guaranteed.
The Regulatory Services are an independent island in the Dr. Brill family because their services can be booked independently of laboratory operations. The efficacy tests required for product approval can, but of course do not have to, be commissioned from Dr. Brill + Partner. However, the short line to the technical expertise of the associated test laboratories and the possibility of commissioning time-critical data packages at short notice within the network can be a decisive advantage for the customer in the approval process.
Dr. Florian Brill, Owner and Managing Director of Dr. Brill Institutes, sums it up: "With the integration of Dr. Brill Regulatory Services GmbH into the Dr. Brill Institutes Group, we have gained a decisive new building block that will help us strategically. We are bringing regulatory expertise in-house. A large part of our work in the various laboratories results in an approval dossier according to BPR, for example, and now we can deliver this. Dr. Brill Regulatory Services was the missing component - and with Gunnar and Heike we have gained two superstars of the scene. I want them in my team and so do our customers. I'm really looking forward to everything else."
In the coming weeks and months, the new Dr. Brill Regulatory Services team will be presenting itself to clients at conferences and presentations. Dr. Gunnar Kleist: "It is important that clients build up a basis of trust with us and also realize what makes us special. That we don't do anything by the book, but always create the best case for the customer. As a rather small team, we deliver what we promise with our personal expertise!"
If you would like more information about our new service, please contact Heike Schimmelpfennig (heike.schimmelpfennig@brillregulatory.com) or Dr. Gunnar Kleist (gunnar.kleist@brillregulatory.com).

Topic "Sustainable hygiene" at the Online Update Hygiene and Infection Prevention #14
As part of the free Online Update #14 on the topic of "Sustainable hygiene", you will have the opportunity to find out about important questions on the topic and exchange ideas with experts from the clinical, research, political and industrial sectors. The online update is a cooperative event with DR. BRILL INSTITUTES, MedWiss4you and HIHeal and will be held in German language. All information on the content and for your registration can be found under the following link:
#14 - Nachhaltige Hygiene

Hydrotox new member of the Dr. Brill Institutes Group
Hydrotox - Labor für Ökotoxikologie und Gewässerschutz GmbH in Freiburg is now part of the Dr. Brill Institutes Group. Hydrotox offers laboratory services on biodegradability, ecotoxicity, genotoxicity and mutagenicity as well as tests to prove algicidal efficacy, advice on environmentally relevant issues and support in the registration and authorization of chemicals and products. You can find the Hydrotox website at www.hydrotox.de. We warmly welcome the entire Hydrotox team under the umbrella of the Dr. Brill Group and are confident that this acquisition will enable us to provide our customers with an even more comprehensive service. In the following the message from Hydrotox:
"We have the pleasure informing you that 35 years after establishing of Hydrotox – Laboratory for Ecotoxicology and Water Protection GmbH, Dr. Stefan Gartiser will retire. The business handover was carefully prepared and completed in January 2024, when Hydrotox GmbH was acquired by Dr. Brill Institutes Group based in Hamburg. The new managing director of Hydrotox GmbH is Dr. Florian H. H. Brill. Your local contact at Hydrotox remains Dr. Christoph Hafner, who is the representative of the local management and has been assigned with the respective power of attorney.
Thus, a new generation takes over at Hydrotox. However, you as our customer will not face apparent changes in the operational processes, as the entire Hydrotox team continues to support you in future with the usual trustworthiness at Freiburg.
The Dr. Brill Institutes Group is a medium-size, family-owned group of companies with the headquarter in Hamburg. The group consists of various laboratories such as the Institute for Hygiene and Microbiology and the Institute for Antifouling and Biocorrosion and employs around 70 people at various locations in Germany. By integrating Hydrotox, synergy effects are released by optimizing and streamlining processes. In addition, the Dr. Brill Institutes Group offers its customers an expanded product portfolio from a wide range of laboratory services to regulatory consulting services in the new Dr. Brill Regulatory Services GmbH. For more information, visit www.brillinstitutes.com.
We look forward continuing our successful cooperation in the future."

Dr. Stefan Gartiser, Dr. Florian H. H. Brill

Arab Health 2024: Please visit us!
Arab Health will take place in the Dubai World Trade Centre from 29 January - 1 February 2024. This time, Dr. Kerstin Walendy-Gnirß will be present. Please contact us for an appointment there and use the opportunity for a personal exchange. Simply send your request by email to info@brillhygiene.com. We would be very pleased to meet you again or to get to know you in Dubai!
At the same time, we would like to thank all of you who visited us at MEDICA 2023 in Düsseldorf in November. It was again a great pleasure for us to maintain, refresh and make so many new contacts there.

Great 9th CAHMV 2023 - 10th anniversary edition this year!
The 9th Conference of Applied Hygiene, Microbiology and Virology last November was once again a great success, and again attracted international attention. Almost 100 people from 16 countries took part - some online, but the majority in person on site. Both days were characterized by lively exchanges and discussions on the presentations and intensive networking during the breaks. Of course, the Keynote Lectures by Leslie Rachel, MSc, USA, Professor Dr. Kurt-Wilhelm Stahl, Freiburg and Dr. Florian H. H. Brill were of particular interest. Another highlight was the first-time cooperation with our partner BioGenius, who contributed a highly interesting session on the topic of regulatory. The event was rounded off with a visit to a wonderful winter Christmas market and a merry dinner on the Alster.
Save the Date: Celebrate with us this year's big anniversary CAHMV, the 10th edition of our conference! On 28 + 29 November 2024, we would like to welcome you again together with BioGenius at the AMERON Hamburg Hotel Speicherstadt for the 10th CAHMV 2024, as well as for a maritime evening program. We would of course be delighted to welcome you. For a non-binding pre-reservation, simply send an e-mail to claudia.steinfath@brillhygiene.com.